Complete guide to nectar and pollen plants of Sabalan region with unique ecosystem analysis, flowering calendar and...
The Role of Honey in Advanced Wound Care: Scientific Evidence, Biological Mechanisms, and Clinical Applications in Modern Medicine
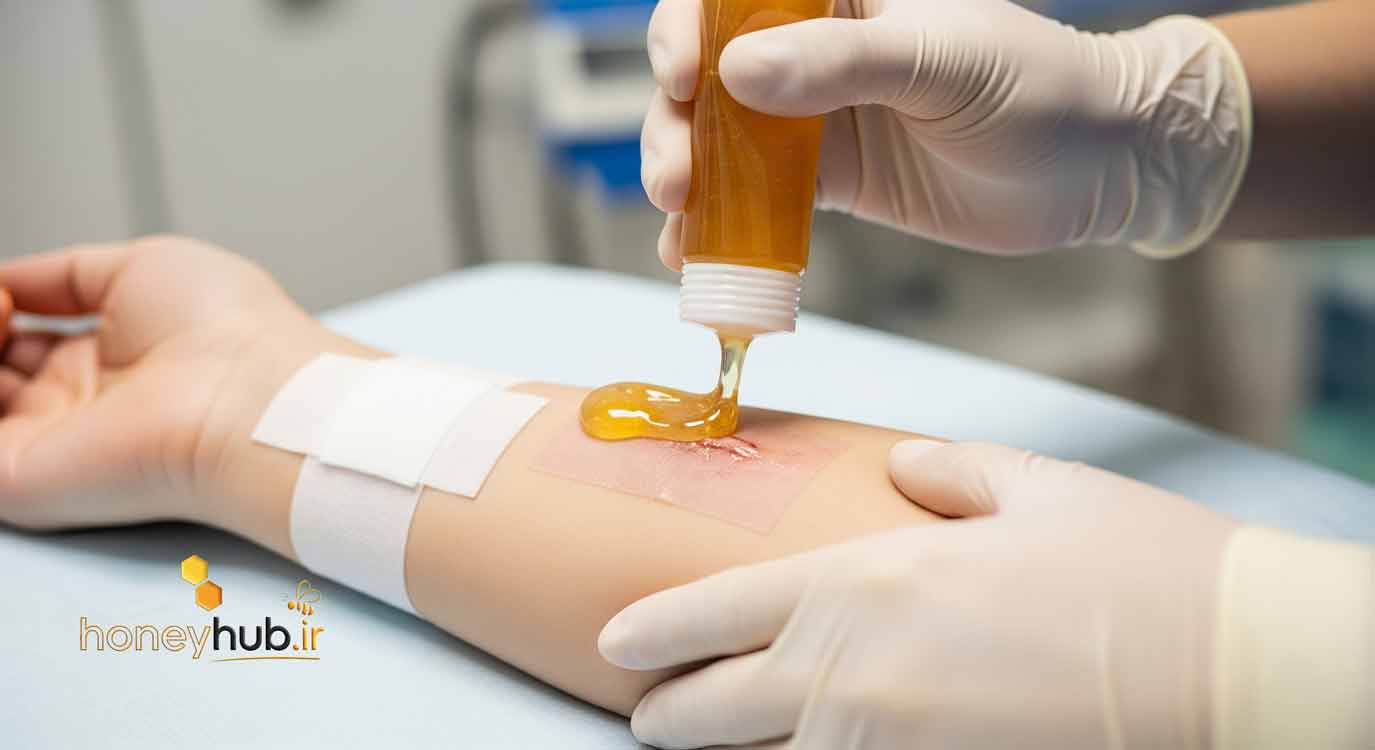
In the era of personalized medicine and biotechnology, a science-backed return to natural remedies is transforming the treatment of chronic and complex wounds. One of the most promising examples is the use of honey as a bioactive agent in advanced wound care. According to a comprehensive systematic review and meta-analysis published in the peer-reviewed journal Molecules in 2021, honey is not just a traditional remedy—it’s a biological therapy with strong clinical evidence supporting its role in accelerating healing for diabetic ulcers, pressure sores, surgical wounds, and burns.
In this article, we dive deep into the biological mechanisms of honey, compare medical-grade varieties, review the latest clinical data, and provide practical, evidence-based guidelines for safe and effective use in modern medical settings.
Honey as a Bioactive Agent: The Science Behind Clinical Use
Honey is a natural substance rich in bioactive compounds, produced by honeybees (Apis mellifera) from floral nectar. Its unique composition—featuring natural sugars (fructose and glucose), enzymes, organic acids, vitamins, minerals, flavonoids, phenolic compounds, and hydrogen peroxide—makes it a multifunctional agent in medicine. Scientific literature confirms that honey supports wound healing through four primary biological mechanisms:
1. Multifaceted Antibacterial Action
Honey demonstrates broad-spectrum activity against Gram-positive bacteria (such as Staphylococcus aureus and Enterococcus faecalis) and Gram-negative pathogens (including Pseudomonas aeruginosa and Escherichia coli). This effect is achieved through a combination of physical and chemical factors:
- Osmotic Effect: High sugar concentration dehydrates bacterial cells and disrupts their metabolism.
- Hydrogen Peroxide: The enzyme glucose oxidase (GOX) in honey slowly releases H₂O₂, providing sustained antibacterial activity.
- Phenolic Compounds and Flavonoids: These damage bacterial cell walls and disrupt membrane function.
- Specialized Compounds like MGO: In Manuka honey, methylglyoxal (MGO) acts as a potent non-peroxide antibacterial agent, effective even against MRSA and VRE.
2. Anti-Inflammatory and Immune-Modulating Effects
Honey reduces levels of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 at the wound site, while boosting production of anti-inflammatory cytokines like IL-10. This immune modulation helps prevent chronic inflammation and supports the transition from the inflammatory phase to the repair phase. Additionally, honey helps regulate neutrophil activation and limits excessive production of reactive oxygen species (ROS), protecting healthy tissue from oxidative damage.
3. Stimulation of Cell Proliferation, Angiogenesis, and Granulation Tissue Formation
Honey enhances the secretion of growth factors such as VEGF (vascular endothelial growth factor), FGF (fibroblast growth factor), and TGF-β (transforming growth factor-beta), promoting key healing processes:
- Mobilization and proliferation of fibroblasts
- Production of Type I and III collagen
- Formation of new blood vessels (angiogenesis)
- Differentiation of keratinocytes and re-epithelialization
These effects accelerate the formation of granulation tissue and wound closure.
4. Moist Wound Environment and Autolytic Debridement
Honey’s hygroscopic nature draws moisture from the surrounding environment, creating a moist yet non-soggy wound bed. This optimal environment:
- Prevents drying of newly formed tissue.
- Enhances the activity of lysozyme and cationic enzymes.
- Facilitates autolytic debridement of necrotic tissue.
- Protects the wound from further contamination.
Comparing Medical-Grade Honey: Composition and Clinical Differences
The therapeutic potency of honey depends heavily on its botanical origin, geographic source, and processing method. Based on recent findings and updated research, the table below provides a detailed comparison of the most widely used medical-grade honeys:
| Honey Type | Antibacterial Strength | Key Active Compound | Primary Mechanism | Recommended Clinical Use | Quality Standard |
|---|---|---|---|---|---|
| Manuka (New Zealand) | +++++ | MGO (Methylglyoxal) | Non-enzymatic, catalase-resistant | Antibiotic-resistant wounds, diabetic ulcers, cavity infections | UMF 10+ or MGO 263+ |
| Tualang (Malaysia) | ++++ | Phenolic compounds (e.g., caffeic acid phenethyl ester) | Enzymatic + non-enzymatic | First- and second-degree burns, surgical wounds | TPC > 200 mg GAE/kg |
| Acacia (Europe) | +++ | Hydrogen Peroxide (active GOX) | Enzymatic (pH- and temperature-dependent) | Superficial wounds, scrapes, skin irritations | Active GOX, H₂O₂ > 200 µM |
| Iranian Wildflower (e.g., Ziziphus, Olive, Jasmine) | +++ to ++++ | Phenolics and flavonoids (quercetin, luteolin) | Enzymatic + non-enzymatic | Superficial wounds, skin inflammation, diaper rash | TPC > 150 mg GAE/kg, confirmed antibacterial activity |
Important Note: For medical use, always choose sterile, standardized honey with verified microbiological safety (free from Clostridium botulinum spores). Raw or non-sterile honey may pose infection or toxicity risks and should never be used on open wounds.
Clinical Evidence and Global Meta-Analyses
According to the Cochrane systematic review (Jull et al., 2015, updated 2022), medical honey use leads to significant improvements compared to conventional dressings:
Faster healing in diabetic foot ulcers (95% CI: 28–52%)
Reduced risk of surgical wound infection
Epithelialization rate in burn wounds
In a randomized controlled trial (RCT) involving 120 patients with diabetic ulcers, the Manuka honey group (UMF 15+) healed 2.7 days faster than the control group using silver sulfadiazine (P < 0.01). Pain scores on the VAS scale were also 40% lower in the honey group (P = 0.003). No severe allergic reactions were reported.
Studies show honey remains effective even in chronic wounds with bacterial biofilms, as its components can disrupt biofilm structure and enhance antibiotic penetration.
Standard Guidelines for Using Honey in Wound Treatment
To maximize efficacy and safety, honey should be applied following clinical best practices:
- Wound Assessment: Evaluate wound type (acute/chronic), depth, presence of infection or biofilm, and patient factors (diabetes, immunosuppression).
- Cleaning: Clean the wound with sterile saline or 0.5% hydrogen peroxide if exudate is present.
- Selecting Honey: Use medical-grade honey with UMF ≥ 15+ or MGO ≥ 500 for deep or infected wounds.
- Application: Apply a thin layer (2–3 mm) of honey to the wound and 1–2 cm around the margins.
- Dressing: Cover with a moist dressing (e.g., sterile gauze or hydrocolloid) to prevent honey from drying.
- Change Frequency: Replace daily or every 12–24 hours, depending on wound exudate levels.
- Monitoring: Check daily for signs of infection, inflammation, or allergic reaction.
Warning: Honey should only be used under the supervision of a wound care specialist for deep, necrotic, or immunocompromised patients. Discontinue use and consult a physician immediately if redness, swelling, increased pain, or unusual discharge occurs.
Challenges and the Future of Honey in Biomedical Medicine
Despite strong evidence, challenges remain in standardization, batch variability, and widespread adoption in healthcare systems. Current research is focused on:
- Developing honey-impregnated dressings
- Combining honey with nanoparticles or biodegradable polymers
- Integrating honey into bioengineered skin grafts
- Identifying biomarkers for treatment response
These innovations aim to enhance honey’s efficacy and solidify its role in advanced medical care.
Conclusion
Honey—especially standardized medical-grade varieties—is a multifunctional bioactive agent with robust clinical evidence for managing complex wounds. Supported by updated research and global studies, honey accelerates wound healing through antibacterial, anti-inflammatory, pro-angiogenic, and moisture-balancing effects.
While honey cannot replace modern treatments like antibiotics or surgery, it serves as a powerful adjuvant therapy that can improve healing outcomes and reduce healthcare costs. The future of honey in medicine lies in its integration with biotechnology and personalized treatment strategies.
Scientific References
- Scepankova, H., Combarros-Fuertes, P., Fresno, J. M., Tornadijo, M. E., Dias, M. S., Pinto, C. A., Saraiva, J. A., & Estevinho, L. M. (2021). Role of Honey in Advanced Wound Care. Molecules, 26(16), 4784. https://doi.org/10.3390/molecules26164784
- Jull, A. B., Cullum, N., Dumville, J. C., Westby, M. J., Deshpande, S., & Walker, N. (2015). Honey as a topical treatment for wounds. Cochrane Database of Systematic Reviews, (3). https://doi.org/10.1002/14651858.CD005083.pub3
- Molan, P. C. (2006). Why honey is effective as a medicine. 2. The scientific explanation of its effects. International Wound Journal, 3(2), 159–171.
- Abuharfeil, N., Al-Oran, R., & Abo Shehada, M. (2019). The effect of bee honey on the immune system. Nutrition Research Reviews, 32(2), 182–195.
- Yaghoobi, N., Kazerouni, A., & Kazerouni, O. (2013). Evidence for using honey in wound healing. Indian Journal of Plastic Surgery, 46(2), 299–306.
- Alvarez-Suarez, J. M., et al. (2020). Antioxidant and antimicrobial properties of honey. Foods, 9(4), 424.























Latest comments